Community Tip - Did you get an answer that solved your problem? Please mark it as an Accepted Solution so others with the same problem can find the answer easily. X
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
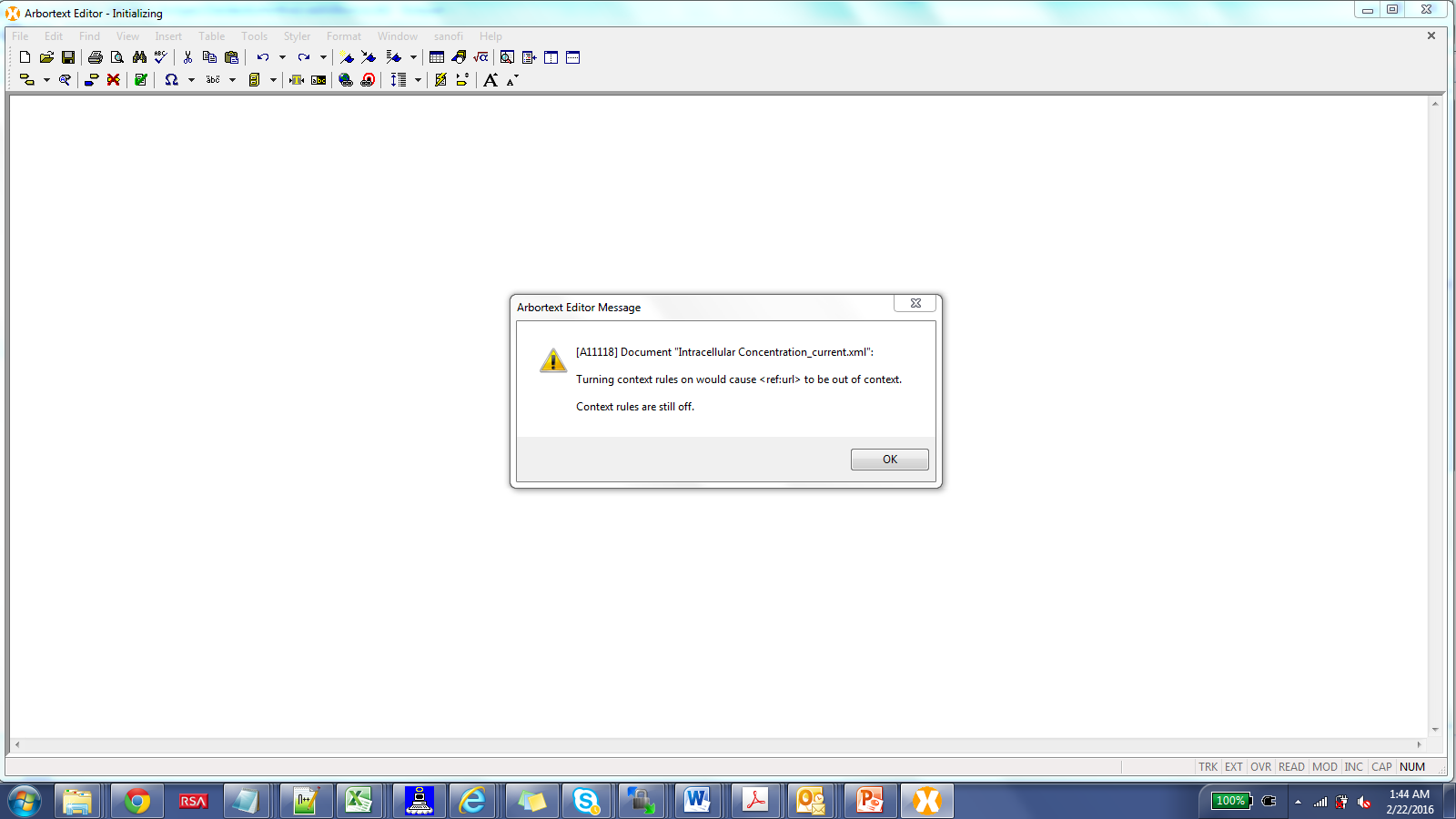
Getting a pop up <ref:url> out of context
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Getting a pop up <ref:url> out of context
<?xml version="1.0" encoding="UTF-8"?>
<!DOCTYPE StandardLetterBody PUBLIC "-//Aventis Pharmaceuticals//Standard Letter Body//EN" "file:C:\Users\NM28079\medinfo\XML_Standard_Letter_Body\StandardLetterBody.dtd">
<StandardLetterBody xmlns:slb="http://www.aventis.com/amis/standardletterbody" xmlns:atict="http://www.arbortext.com/namespace/atict"><atict:info color="" print="" ref="0" tracking="off"/><atict:user fullname="Bell, Mark" user="nm60913"/>
<slb:DocumentProperties>
<DocumentTitle>Intracellular Concentration</DocumentTitle>
<TherapeuticArea>Respiratory</TherapeuticArea>
<GenericName>telithromycin</GenericName>
<TradeName>Ketek</TradeName>
<DocumentSubject>Pharmacokinetics-Pharmacodynamics</DocumentSubject>
<SubjectCategory/>
<SubjectSubCategory/>
<ChangeSummary>Final, added Barcia-Macay reference</ChangeSummary>
</slb:DocumentProperties>
<slb:DocumentContent>
<sect1>
<title>As stated in the KETEK package insert:<ref:reference confidentiality="Public" object_id="09012bda8001f259" xmlns:ref="ref"><ref:packageinsert><ref:tradename>Ketek</ref:tradename><ref:companylocation>Bridgewater, NJ</ref:companylocation><ref:companyname>sanofi-aventis U.S.</ref:companyname><ref:publicationdate>2010</ref:publicationdate></ref:packageinsert></ref:reference></title>
<blockquote>
<para><emphasis role="bold">CLINICAL PHARMACOLOGY</emphasis></para>
<para><emphasis role="bold"><emphasis role="underline">Pharmacokinetics</emphasis></emphasis></para>
<para><emphasis role="bold">Distribution:</emphasis></para>
<para>Telithromycin concentrations in ...alveolar macrophages after
800 mg once daily dosing for 5 days in patients are displayedin Table
2.</para>
<informaltable>
<tgroup cols="5">
<?PubTbl tgroup dispwid="6.64in"?>
<colspec colname="col1" colwidth="1.60*"/><colspec colname="col2" colwidth="0.85*"/><colspec colname="col3" colwidth="0.77*"/><colspec colname="col4" colwidth="0.84*"/><colspec colname="col5" colwidth="0.97*"/>
<tbody>
<row>
<entry nameend="col5" namest="col1" rowsep="0"><emphasis role="bold">Table 2</emphasis></entry>
</row>
<row>
<entry colsep="0" nameend="col2" namest="col1"><emphasis role="bold"/></entry>
<entry colsep="1" nameend="col5" namest="col3">Mean concentration
(µg/mL)</entry>
</row>
<row>
<entry/>
<entry><para><emphasis role="bold">Hours</emphasis><?Pub _newline
?><emphasis role="bold">post-</emphasis><?Pub _newline?><emphasis role="bold">dose</emphasis></para></entry>
<entry valign="bottom"><para><emphasis role="bold">Tissue or</emphasis><?Pub _newline?><emphasis role="bold">fluid</emphasis></para></entry>
<entry valign="bottom"><para><emphasis role="bold">Plasma</emphasis></para></entry>
<entry><para><emphasis role="bold">Tissue/</emphasis><?Pub _newline
?><emphasis role="bold">Plasma</emphasis><?Pub _newline?><emphasis role="bold">Ratio</emphasis></para></entry>
</row>
<row>
<entry>Alveolar macrophages</entry>
<entry align="center"><para>2<?Pub _newline?>8<?Pub _newline?>24</para></entry>
<entry align="center"><para>65<?Pub _newline?>100<?Pub _newline?>41</para></entry>
<entry align="center"><para>1.07<?Pub _newline?>0.605<?Pub _newline
?>0.073</para></entry>
<entry align="center"><para>55<?Pub _newline?>180<?Pub _newline?>540</para></entry>
</row>
</tbody>
</tgroup>
</informaltable>
<para>Telithromycin concentration in white blood cells exceeds the
concentration in plasma and is eliminated more slowly from white blood
cells than from plasma. Mean white blood cell concentrations of telithromycin
peaked at 72.1 µg/mL at 6 hours, and remained at 14.1 µg/mL
24 hours after 5 days of repeated dosing of 600 mg once daily. After
10 days, repeated dosing of 600 mg once daily, white blood cell concentrations
remained at 8.9 µg/mL 48 hours after the last dose.</para>
<para><emphasis role="underline"><emphasis role="bold">Microbiology</emphasis></emphasis></para>
<para>...Telithromycin concentrates in phagocytes where it exhibits
activity against intracellular respiratory pathogens...</para>
</blockquote>
<para>Telithromycin, a ketolide<ref:reference confidentiality="Public" object_id="09012bda8001b69f" xmlns:ref="ref"><ref:journalarticle><ref:author>Blaney SM</ref:author><ref:author>Seibel NL</ref:author><ref:author>O'Brien M</ref:author><ref:author>Reaman GH</ref:author><ref:author>Berg SL</ref:author><ref:author>Adamson PC</ref:author><ref:author>Poplack DG</ref:author><ref:author>Krailo MD</ref:author><ref:author>Mosher R</ref:author><ref:author>Balis FM</ref:author><ref:journaltitle>Phase I trial of docetaxel administered as a 1-hour infusion in children with refractory solid tumors: a collaborative Pediatric Branch, National Cancer Institute and Children's Cancer Group trial</ref:journaltitle><ref:serialtitle>J Clin Oncol</ref:serialtitle><ref:publicationdate>1997</ref:publicationdate><ref:volume>15</ref:volume><ref:issuenumber>4</ref:issuenumber><ref:firstpage>1538</ref:firstpage><ref:lastpage>1543</ref:lastpage></ref:journalarticle></ref:reference> having a keto-group rather than
a sugar (L-cladinose) at position 3 of the lactone ring, is a derivative
of the macrolide antibiotics. Macrolide antibiotics concentrate within
host cells, a characteristic responsible for activity against intracellular
pathogens and modulation of cell metabolism and function.<ref:reference confidentiality="Public" object_id="09012bda8001f8af" xmlns:ref="ref"><ref:journalarticle><ref:author>Labro MT</ref:author><ref:author>Abdelghaffar H</ref:author><ref:journaltitle>Immunomodulation by macrolide antibiotics</ref:journaltitle><ref:serialtitle>J Chemother</ref:serialtitle><ref:publicationdate>2001</ref:publicationdate><ref:volume>13</ref:volume><ref:issuenumber>1</ref:issuenumber><ref:firstpage>3</ref:firstpage><ref:lastpage>8</ref:lastpage></ref:journalarticle></ref:reference> Telithromycin
has been documented to accumulate within various cells.<ref:reference confidentiality="Confidential" object_id="09012bda8001f3fa" xmlns:ref="ref"><ref:studyreport><ref:studyreportnumber>NDA 21-144 Original</ref:studyreportnumber></ref:studyreport></ref:reference> <hiddentext xmlns:ref="ref">(NDA Section 7.B.1.2.5.4, 7:v001:p172–176)</hiddentext> At an extracellular concentration of 2.5 µg/mL, telithromycin
gradually accumulates in polymorphonuclear neutrophils (PMNs) with
an intracellular/extracellular concentration ratio (C/E) ranging from
27.0 ± 8.1 (at 5 minutes) to 348 ± 27.1 (at 160 minutes).
At an extracellular concentration of 2 µg/mL, telithromycin
uptake into macrophages is also rapid with a C/E ratio of 65 (at 60
minutes). </para><?Pub Caret 25?>
</sect1>
<sect1>
<title>Intracellular Kinetics of Telithromycin</title>
<para>The ability of telithromycin to enter PMNs, peripheral blood
mononuclear cells and cells of hematopoietic and nonhematopoietic
origin was investigated <emphasis role="italic">in vitro</emphasis>.<ref:reference confidentiality="Public" object_id="09012bda8001f8b1" xmlns:ref="ref"><ref:journalarticle><ref:author>Miossec-Bartoli C</ref:author><ref:author>Pilatre L</ref:author><ref:author>Peyron P</ref:author><ref:author>N'Diaye EN</ref:author><ref:author>Collart-Dutilleul V</ref:author><ref:author>Maridonneau-Parini I</ref:author><ref:author>Diu-Hercend A</ref:author><ref:journaltitle>The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells.</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>1999</ref:publicationdate><ref:volume>43</ref:volume><ref:issuenumber>10</ref:issuenumber><ref:firstpage>2457</ref:firstpage><ref:lastpage>2462</ref:lastpage></ref:journalarticle></ref:reference> Telithromycin was slowly taken up into PMNs achieving a C/E concentration
ratio around 300 after 2 hours. It concentrated and remained within
the cells, with 80% of the drug remaining cell associated after 2
hours. Subcellular fractionation of the PMNs showed that the majority
of telithromycin resides within the azurophil granules. Incorporation
of telithromycin into other cell types (peripheral blood mononuclear
cells, T-lymphocytic cells, monocytic cells, erythroid progenitor
cell types, promyelocytic cell line and colon carcinoma cell line)
was poor with quick release.</para>
<para>In order for an antimicrobial agent to be effectively transported
to a location of infection, it must not interfere with the ability
of the neutrophil to sense chemoattractants and to move normally.
A study was performed to evaluate the effect of telithromycin on
these properties in an <emphasis role="italic">in vitro</emphasis> model utilizing <emphasis role="italic">Streptococcus pyogenes</emphasis>, <emphasis role="italic">Staphylococcus aureus</emphasis>, <emphasis role="italic">Escherichia coli</emphasis>, and <emphasis role="italic">Micrococcus luteus</emphasis>.<ref:reference confidentiality="Public" object_id="09012bda8001f8b3" xmlns:ref="ref"><ref:journalarticle><ref:author>Mandell GL</ref:author><ref:author>Coleman E</ref:author><ref:journaltitle>Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils.</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>2001</ref:publicationdate><ref:volume>45</ref:volume><ref:issuenumber>6</ref:issuenumber><ref:firstpage>1794</ref:firstpage><ref:lastpage>1798</ref:lastpage></ref:journalarticle></ref:reference> Among the agents tested (penicillin G, azithromycin, ciprofloxacin,
levofloxacin, and moxifloxacin), telithromycin was documented to be
highly concentrated and slowly released from PMNs. Telithromycin
was transported by PMNs towards a chemoattractant, resulting in zones
of bacterial inhibition that were greater than those reported for
the other agents tested. </para>
<para>The intracellular kinetics of telithromycin, its effects on
pro-inflammatory cytokines, and its effect on PMN bactericidal activity
was examined in a series of<emphasis role="italic"> in vitro</emphasis> studies. Telithromycin was shown to concentrate in PMNs displaying
a C/E concentration ratio of 31 ± 4.2 at 5 minutes up to 348
± 27.1 at 180 minutes.<ref:reference confidentiality="Public" object_id="09012bda8001f81c" xmlns:ref="ref"><ref:journalarticle><ref:author>Vazifeh D</ref:author><ref:author>Preira A</ref:author><ref:author>Bryskier A</ref:author><ref:author>Labro MT</ref:author><ref:journaltitle>Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>1998</ref:publicationdate><ref:volume>42</ref:volume><ref:issuenumber>8</ref:issuenumber><ref:firstpage>1944</ref:firstpage><ref:lastpage>1951</ref:lastpage></ref:journalarticle></ref:reference> It was noted
that approximately 60% of telithromycin was concentrated in the granular
compartment of PMNs and the intracellular uptake displayed Michaelis-Menten
saturation kinetics. Uptake was dependent on environmental temperatures
and extracellular calcium was necessary for optimal uptake. Telithromycin
weakly triggered granule exocytosis from PMNs and inhibited superoxide
anion production (oxidative burst) in a time and concentration-dependent
manner. </para>
<para>The effects of various proinflammatory cytokines (interleukin
1 [IL-1], IL-6, IL-8, gamma interferon, tumor necrosis factor alpha
(TNF-a) and granulocyte-macrophage colony stimulating factor (GM-CSF)
on cellular uptake of telithromycin and the interaction of telithromycin
with polymorphonuclear neutrophils (PMN) has been investigated.<ref:reference confidentiality="Public" object_id="09012bda8001bcf7" xmlns:ref="ref"><ref:journalarticle><ref:author>Vazifeh D</ref:author><ref:author>Bryskier A</ref:author><ref:author>Labro MT</ref:author><ref:journaltitle>Effect of proinflammatory cytokines on the interplay between roxithromycin, HMR 3647, or HMR 3004 and human polymorphonuclear neutrophils</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>2000</ref:publicationdate><ref:volume>44</ref:volume><ref:issuenumber>3</ref:issuenumber><ref:firstpage>511</ref:firstpage><ref:lastpage>521</ref:lastpage></ref:journalarticle></ref:reference> In this study, TNF-a and GM-CSF were the only cytokines to exert
an effect on telithromycin, lessening the inhibitory effects of telithromycin
on PMN oxidant production. Inhibitory concentrations suggest that
telithromycin acts downstream of the priming effect of TNF-a and GM-CSF.
TNF-a and GM-CSF also modestly decreased the PMN uptake of telithromycin
by approximately 20%.</para>
<para>In the final study, the effects of telithromycin on bactericidal
activity of PMNs was studied utilizing four strains of Staphylococcus
aureus with different profiles of susceptibility to macrolides and
ketolides.<ref:reference confidentiality="Public" object_id="09012bda8001f8b5" xmlns:ref="ref"><ref:journalarticle><ref:author>Vazifeh D</ref:author><ref:author>Abdelghaffar H</ref:author><ref:author>Labro MT</ref:author><ref:journaltitle>Effect of telithromycin (HMR 3647) on polymorphonuclear neutrophil killing of Staphylococcus aureus in comparison with roxithromycin.</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>2002</ref:publicationdate><ref:volume>46</ref:volume><ref:issuenumber>5</ref:issuenumber><ref:firstpage>1364</ref:firstpage><ref:lastpage>1374</ref:lastpage><ref:url>www.ggggle.com</ref:url></ref:journalarticle></ref:reference> The effects of intracellular bacteria on cellular uptake of telithromycin
was also evaluated. High concentrations of telithromycin, which abolished
the oxidative metabolism of the PMNs, did not impair bactericidal
function. In a global assay, the bactericidal activity of telithromycin
was not impaired in the presence of PMNs. The authors concluded that
the antibacterial activity of telithromycin against <emphasis role="italic" xmlns:ref="ref">S. aureus</emphasis> in this setting
was a direct effect of the drug, and not due to a synergistic interaction
with the bactericidal system of the PMNs. </para>
<para>The activity of telithromycin against intracellular <emphasis role="italic">Legionella pneumophilia</emphasis> was investigated
in an <emphasis role="italic">in vitro</emphasis> study using human
monocytes.<ref:reference confidentiality="Public" object_id="09012bda8001f8b6" xmlns:ref="ref"><ref:journalarticle><ref:author>Baltch AL</ref:author><ref:author>Smith RP</ref:author><ref:author>Ritz WJ</ref:author><ref:author>Franke MA</ref:author><ref:author>Michelsen PB</ref:author><ref:journaltitle>Antibacterial effect of telithromycin (HMR 3647) and comparative antibiotics against intracellular Legionella pneumophila.</ref:journaltitle><ref:serialtitle>J Antimicrob Chemother</ref:serialtitle><ref:publicationdate>2000</ref:publicationdate><ref:volume>46</ref:volume><ref:issuenumber>1</ref:issuenumber><ref:firstpage>51</ref:firstpage><ref:lastpage>55</ref:lastpage></ref:journalarticle></ref:reference> Telithromycin (10X MIC for 4 days) produced a significant antimicrobial
effect on monocytes exposed to <emphasis role="italic" xmlns:ref="ref">L. pneumophlia</emphasis>. There were no significant differences
observed with regards to the growth of intracellular <emphasis role="italic" xmlns:ref="ref">L. pneumophilia</emphasis> on days 2-4
when telithromycin was removed following 1 day of exposure. The authors
noted that removal of telithromycin did not affect the continued antimicrobial
activity of the monocytes. </para>
<para>Telithromycin's intracellular activity against <emphasis role="italic">S. aureus </emphasis> ATCC 25493 (MIC 2 mg/L) was evaluated
in human THP-1 macrophages infection model.<ref:reference confidentiality="Public" object_id="09012bda80020c90" xmlns:ref="ref"><ref:abstract><ref:author>Barcia-Macay M</ref:author><ref:author>Seral C</ref:author><ref:author>Mingeot-Leclercq MP</ref:author><ref:author>Tulkens PM</ref:author><ref:abstracttitle>Comparative activity of 12 antibiotics (ABs) used at clinically-meaningful extracellular concentration against S. aureus in broth and in human THP-1 macrophages</ref:abstracttitle><ref:conference>43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, September 14-17, 2003</ref:conference><ref:abstractnumber>A-1174</ref:abstractnumber></ref:abstract></ref:reference> At a concentration of 2 mg/L, telithromycin's
extracellular and intracellular activities were -1.84±0.02
log change in cfu in 24 hours in broth and –1.14±0.04
log change in cfu in 24 hours in human THP-1 macrophages, respectively.</para>
<para>A study evaluated the effect of P-glycoprotein inhibitors (cyclosporin
A, verapamil, GF120918) on the accumulation and efflux of telithromycin
in J774 murine macrophages.<ref:reference confidentiality="Public" object_id="09012bda8001fd82" xmlns:ref="ref"><ref:journalarticle><ref:author>Seral C</ref:author><ref:author>Michot J-M</ref:author><ref:author>Chanteux H</ref:author><ref:author>Mingeot-Leclercq M-P</ref:author><ref:author>Tulkens PM</ref:author><ref:author>Van Bambeke F</ref:author><ref:journaltitle>Influence of p-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>2003</ref:publicationdate><ref:volume>47</ref:volume><ref:issuenumber>3</ref:issuenumber><ref:firstpage>1047</ref:firstpage><ref:lastpage>1051</ref:lastpage></ref:journalarticle></ref:reference> In the presence of the inhibitors, there was a 2– to 3.5–fold
increase in the rate of telithromycin accumulation in the macrophages;
however, the efflux of telithromycin from the macrophages was slowed
marginally. Cyclosporin and GF120918 increased the accumulation of
telithromycin in the macrophages by 3– to 4–fold following
3 hours of cell incubation with extracellular telithromycin (5 mg/L).
The effect of verapamil on telithromycin was lower than the effect
of the other inhibitors (P < 0.05). The authors concluded that
the data strongly suggests that modulation of P-glycoprotein is responsible
for the observed effects on the accumulation of telithromycin. They
stated that the conclusions for the study were limited to J774 murine
macrophages and could not be applied to other phagocytic cells. The
effects of the efflux proteins on the intracellular activity of telithromycin
may need to be studied systematically.</para>
</sect1>
<sect1>
<title>Penetration of Telithromycin into WBCs and Alveolar Macrophages</title>
<para>The intracellular accumulation and concentration of telithromycin
in white blood cells (WBC) was studied in blood samples obtained from
healthy male volunteers.<ref:reference confidentiality="Public" object_id="09012bda8001f808" xmlns:ref="ref"><ref:poster><ref:author>Gia HP</ref:author><ref:author>Roeder V</ref:author><ref:author>Namour F</ref:author><ref:author>Sultan E</ref:author><ref:author>Lenfant B</ref:author><ref:postertitle>Telithromycin (HMR 3647) achieves high and sustained concentrations in white blood cells in man</ref:postertitle><ref:conference>5th International Conference on Macrolides, Azalides, Streptogramins, Ketolides, and Oxazolidinones, Seville, Spain, January 26-28, 2000</ref:conference><ref:posternumber>09.27</ref:posternumber></ref:poster></ref:reference> Following administration of either a
single oral dose of telithromycin 600 mg or the same dose administered
once daily for 10 days, WBC and plasma concentrations of telithromycin
as well as the WBC concentration/plasma concentration ratio and area
under the concentration-time curve (AUC) in WBCs and plasma were assessed.
It was reported that telithromycin rapidly concentrates in WBCs following
a single oral dose, reaching 44-fold greater levels than in plasma
1 hour after dosing and increasing to 705 at 24 hours post-dose.
The WBC/plasma concentration ratio ranged from 87 at 2 hours post-dose
on Day 5 to 2201 at 48 hours post-dose on Day 10. The ratio of the
AUC (0-24 hours) for WBC to the AUC(0-24 hours) for plasma on Day
10 of the multiple dose study was 242. The telithromycin concentration
in WBCs exceeded the minimum inhibitory concentration of respiratory
pathogens up to 48 hours following multiple dosing. The authors concluded
that the data suggest a role for telithromycin against intracellular
pathogens.</para>
<para>The penetration of telithromycin into bronchopulmonary tissues
(alveolar macrophages (AM) and epithelial lining fluid (ELF)) was
evaluated in healthy male volunteers.<ref:reference confidentiality="Public" object_id="09012bda8001f7cc" xmlns:ref="ref"><ref:journalarticle><ref:author>Muller-Serieys C</ref:author><ref:author>Soler P</ref:author><ref:author>Cantalloube C</ref:author><ref:author>Lemaitre F</ref:author><ref:author>Gia HP</ref:author><ref:author>Brunner F</ref:author><ref:author>Andremont A</ref:author><ref:journaltitle>Bronchpulmonary disposition of the ketolide telithromycin (HMR 3647)</ref:journaltitle><ref:serialtitle>Antimicrob Agents Chemother</ref:serialtitle><ref:publicationdate>2001</ref:publicationdate><ref:volume>45</ref:volume><ref:issuenumber>11</ref:issuenumber><ref:firstpage>3104</ref:firstpage><ref:lastpage>3108</ref:lastpage></ref:journalarticle></ref:reference> Following a 5–day course of oral telithromycin 800 mg once
daily, patients underwent fiberoptic bronchoscopy and bronchoalveolar
lavage at 2, 8, 24, or 48 hours. Concentrations of telithromycin
in both AM and ELF were significantly greater than those in plasma
at each time point (P<0.05). Telithromycin was retained in AM
24 hours after dosing and was quantifiable 48 hours after dosing.
The authors concluded that while the data were obtained from noninfected
subjects, it was unlikely that infection would reduce the concentration
of telithromycin in AM and ELF.</para>
<para>In a study of 20 patients undergoing elective bronchoscopy,
the mean concentration of telithromycin in alveolar macrophages (AM),
epithelial lining fluid (ELF), and bronchial mucosal (BM) were measured
2, 12, and 24 hours after the last dose of a 5–day course of
oral telithromycin 800 mg once daily.<ref:reference confidentiality="Public" object_id="09012bda8001b980" xmlns:ref="ref"><ref:journalarticle><ref:author>Khair OA</ref:author><ref:author>Andrews JM</ref:author><ref:author>Honeybourne D</ref:author><ref:author>Jevons G</ref:author><ref:author>Vacheron F</ref:author><ref:author>Wise R</ref:author><ref:journaltitle>Lung concentrations of telithromycin after oral dosing</ref:journaltitle><ref:serialtitle>J Antimicrob Chemother</ref:serialtitle><ref:publicationdate>2001</ref:publicationdate><ref:volume>47</ref:volume><ref:issuenumber>6</ref:issuenumber><ref:firstpage>837</ref:firstpage><ref:lastpage>840</ref:lastpage></ref:journalarticle></ref:reference> Telithromycin concentrations
in AM, ELF, and BM were higher (161.57 mg/L, 0.97 mg/L, and 0.78 mg/L,
respectively) than the plasma concentration (0.08 mg/L) at 24 hours
post-dose. At 24 hours post dose, the concentration of telithromycin
in BM and ELF was greater than the MIC90 for the common respiratory
pathogens, <emphasis role="italic" xmlns:ref="ref">Streptococcus pneumoniae</emphasis> (0.12 mg/L) and <emphasis role="italic" xmlns:ref="ref">Moraxella
catarrhalis</emphasis> (0.03 mg/L) as well as the atypical pathogen <emphasis role="italic" xmlns:ref="ref">Mycoplasma pneumoniae</emphasis> (0.001
mg/L). The telithromycin concentration in BM and ELF exceeded the
MIC90 for <emphasis role="italic" xmlns:ref="ref">Haemophilus influenzae</emphasis> (2 mg/L) for 12 hours.</para>
</sect1>
</slb:DocumentContent>
<slb:SupportMaterial>
<EnclosureList><enc:reference confidentiality="Public" object_id="09012bda8001f259" xmlns:enc="enc"><enc:packageinsert><enc:tradename>Ketek</enc:tradename><enc:companylocation>Bridgewater, NJ</enc:companylocation><enc:companyname>sanofi-aventis U.S.</enc:companyname><enc:publicationdate>2010</enc:publicationdate></enc:packageinsert></enc:reference></EnclosureList>
<EnclosureListPlaceHolder/>
<ReferenceListPlaceHolder/><AddReferenceList/>
<AddReferenceListPlaceHolder/></slb:SupportMaterial>
</StandardLetterBody><?Pub *0000024884?>
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Is ref:url defined in the DTD for the ref namespace?
I see it used in your document as
<ref:url>www.ggggle.com</ref:url>
The xml syntax look correct.
What is the definition of the content of ref:url? CDATA?
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Thanks for your response.
ref:url is mentioned as #PCDATA
Following is the DTD:
<?xml version='1.0' encoding='UTF-8' ?>
<!--Generated by XML Authority-->
<!ENTITY % hidden SYSTEM "hidden.dtd">
%hidden;
<!ELEMENT ref:reference ((ref:journalarticle | ref:book | ref:bookchapter | ref:abstract | ref:poster | ref:studyreport | ref:pressrelease | ref:monographpamphlet | ref:website | ref:miscellaneous | ref:literaturesearch | ref:msds | ref:packageinsert | ref:cdromslidekit),ref:hiddenreferences*)>
<!ATTLIST ref:reference xmlns:ref CDATA #FIXED "ref"
object_id CDATA #IMPLIED
confidentiality CDATA #IMPLIED
language CDATA #IMPLIED
therapeuticarea CDATA #IMPLIED
genericname CDATA #IMPLIED
>
<!ELEMENT ref:journalarticle (ref:author* , ref:journaltitle? , ref:serialtitle? , ref:publicationdate? , ref:url? , ref:volume? , ref:issuenumber? , ref:firstpage? , ref:lastpage?)>
<!ELEMENT ref:book (ref:author* , ref:booktitle? , ref:editor* , ref:volumenumber? , ref:volumetitle? , ref:edition? , ref:publisherlocation? , ref:publisher? , ref:publicationdate? , ref:url? )>
<!ELEMENT ref:bookchapter (ref:author* , ref:chaptertitle? , ref:editor* , ref:booktitle? , ref:volumetitle? , ref:edition? , ref:publisherlocation? , ref:publisher? , ref:publicationdate? , ref:url? , ref:firstpage? , ref:lastpage?)>
<!ELEMENT ref:abstract (ref:author* , ref:abstracttitle? , ((ref:serialtitle, ref:publicationdate?) | ref:conference)? , ref:url? , ref:volume? , ref:issuenumber? , ref:firstpage? , ref:lastpage? , ref:abstractnumber?)>
<!ELEMENT ref:poster (ref:author* , ref:postertitle? , ((ref:serialtitle, ref:publicationdate?) | ref:conference)? , ref:url? , ref:volume? , ref:issuenumber? , ref:firstpage? , ref:lastpage? , ref:posternumber?)>
<!ELEMENT ref:studyreport (ref:studyreportnumber?, ref:url? )>
<!ELEMENT ref:pressrelease (ref:author* , ref:referencecontent?, ref:url? )>
<!ELEMENT ref:monographpamphlet (ref:title?, ref:companyname?, ref:publicationdate? , ref:url? , ref:advertisingcode?)>
<!ELEMENT ref:website (ref:author* , ref:title? , ref:url? , ref:accessdate?)>
<!ELEMENT ref:miscellaneous (ref:referencecontent?, ref:url? )>
<!ELEMENT ref:literaturesearch (ref:title? , ref:searchdate?, ref:url? )>
<!ELEMENT ref:msds (ref:tradename? , ref:msdsdate?, ref:msdsdate?, ref:url? )>
<!ELEMENT ref:cdromslidekit (ref:title? , ref:companyname? , ref:publicationdate?, ref:url? )>
<!ELEMENT ref:packageinsert (ref:tradename?, ref:companylocation?, ref:companyname? , ref:publicationdate?, ref:url? )>
<!ELEMENT ref:author (#PCDATA)>
<!ELEMENT ref:journaltitle (#PCDATA)>
<!ELEMENT ref:serialtitle (#PCDATA)>
<!ELEMENT ref:publicationdate (#PCDATA)>
<!ELEMENT ref:volume (#PCDATA)>
<!ELEMENT ref:issuenumber (#PCDATA)>
<!ELEMENT ref:firstpage (#PCDATA)>
<!ELEMENT ref:lastpage (#PCDATA)>
<!ELEMENT ref:booktitle (#PCDATA)>
<!ELEMENT ref:editor (#PCDATA)>
<!ELEMENT ref:volumenumber (#PCDATA)>
<!ELEMENT ref:volumetitle (#PCDATA)>
<!ELEMENT ref:edition (#PCDATA)>
<!ELEMENT ref:publisherlocation (#PCDATA)>
<!ELEMENT ref:publisher (#PCDATA)>
<!ELEMENT ref:chaptertitle (#PCDATA)>
<!ELEMENT ref:abstracttitle (#PCDATA)>
<!ELEMENT ref:conference (#PCDATA)>
<!ELEMENT ref:abstractnumber (#PCDATA)>
<!ELEMENT ref:postertitle (#PCDATA)>
<!ELEMENT ref:studyreportnumber (#PCDATA)>
<!ELEMENT ref:referencecontent (#PCDATA)>
<!ELEMENT ref:url (#PCDATA)>
<!ELEMENT ref:accessdate (#PCDATA)>
<!ELEMENT ref:searchdate (#PCDATA)>
<!ELEMENT ref:msdsdate (#PCDATA)>
<!ELEMENT ref:tradename (#PCDATA)>
<!ELEMENT ref:companyname (#PCDATA)>
<!ELEMENT ref:title (#PCDATA)>
<!ELEMENT ref:advertisingcode (#PCDATA)>
<!ELEMENT ref:posternumber (#PCDATA)>
<!ELEMENT ref:companylocation (#PCDATA)>
<!ELEMENT ref:hiddenreferences (hid:reference+)>
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Your DTD does not allow a <ref:url> to follow a <ref:firstpage> or <ref:lastpage> as seen in
</ref:issuenumber><ref:firstpage>1364</ref:firstpage><ref:lastpage>1374</ref:lastpage><ref:url>www.ggggle.com</ref:url></ref:journalarticle></ref:reference>
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Mandakini,
Were you able to resolve this with Tim's comments?





