Community Tip - Have a PTC product question you need answered fast? Chances are someone has asked it before. Learn about the community search. X
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
Second-Order Reversible Reactions
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Second-Order Reversible Reactions
It is known that for a second-order reversible reactions 8 kinetic mechanisms are possible:
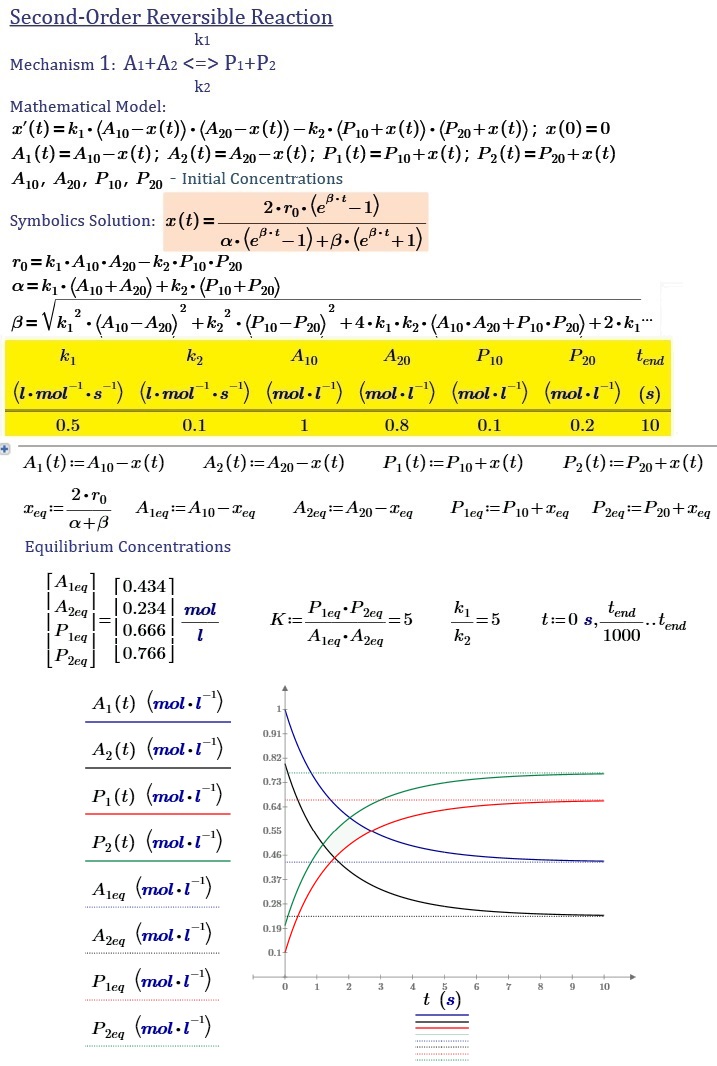
1) A1 + A2 <-> P1 + P2
2) A1 + A2 <-> 2 P
3) 2 A <-> P1 + P2
4) 2 A <-> 2 P
5) A1 + A2 <-> P
6) 2 A <-> P
7) A <-> P1 + P2
😎 A <-> 2 P
Here, A - reagent B - product.
I found a general solution for all the mechanisms that can be represented relatively simple formula
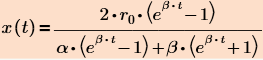
Where r.o - the initial reaction rate, alpha and beta - parameters that are dependent on the rate constants of the forward and reverse reactions and the initial concentrations of the reactants.
For each separate mechanism, these parameters have different values.
Accordingly, I want to make changes in the second edition of the material with respect to the second-order reversible reactions.
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Оказывается, мой подход к анализу обратимых реакций второго порядка правильный.
См. дискуссию по механизму 5 на research.net:
Any literature reference for the kinetics of reversible bimolecular association? - ResearchGate





