Community Tip - Did you know you can set a signature that will be added to all your posts? Set it here! X
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
Rydberg's constant in mathcad 15 is wrong
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Rydberg's constant in mathcad 15 is wrong
- Labels:
-
Other
Accepted Solutions
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Presently the speed of light is defined as (exactly!): 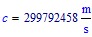 and
and
the unperturbed ground state hyperfine transition frequency of the caesium 133 atom is (exactly): 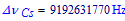
As of (sometime) next year (see http://www.bipm.org/utils/common/pdf/si-brochure-draft-2016b.pdf)
the following definitions will apply (and they will all be exact; their final exact values need to be determined, but they will be close to):
Plank constant:
Electron charge: 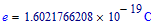
Boltzmann constant: 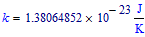
Avogadro constant: 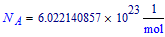
The luminous efficacy Kcd of monochromatic radiation of frequency 540 ×10^12 hertz: 
Further is determined (not exact):

With those, other natural constants become:

Luc
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
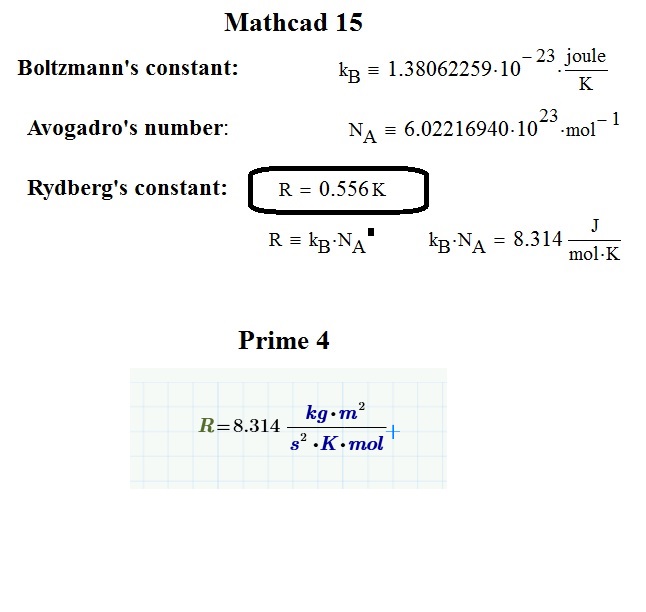
With R you probably have hit the Rankine temperature unit defined in Mathcad 15. The Rankine scale starts at absolute 0 (that is 0 K) and steps in units of the Fahrenheit scale..
So Rankine relates to Fahrenheit as Kelvin relates to Celsius.
The Rydberg constant is written and known as follows:
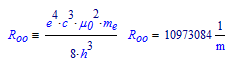
The value commonly denoted with R, and that has a value of about 8.314, is the Universal gas constant:
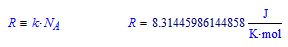
Success!
Luc
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
I apologize, I did some confusion ...
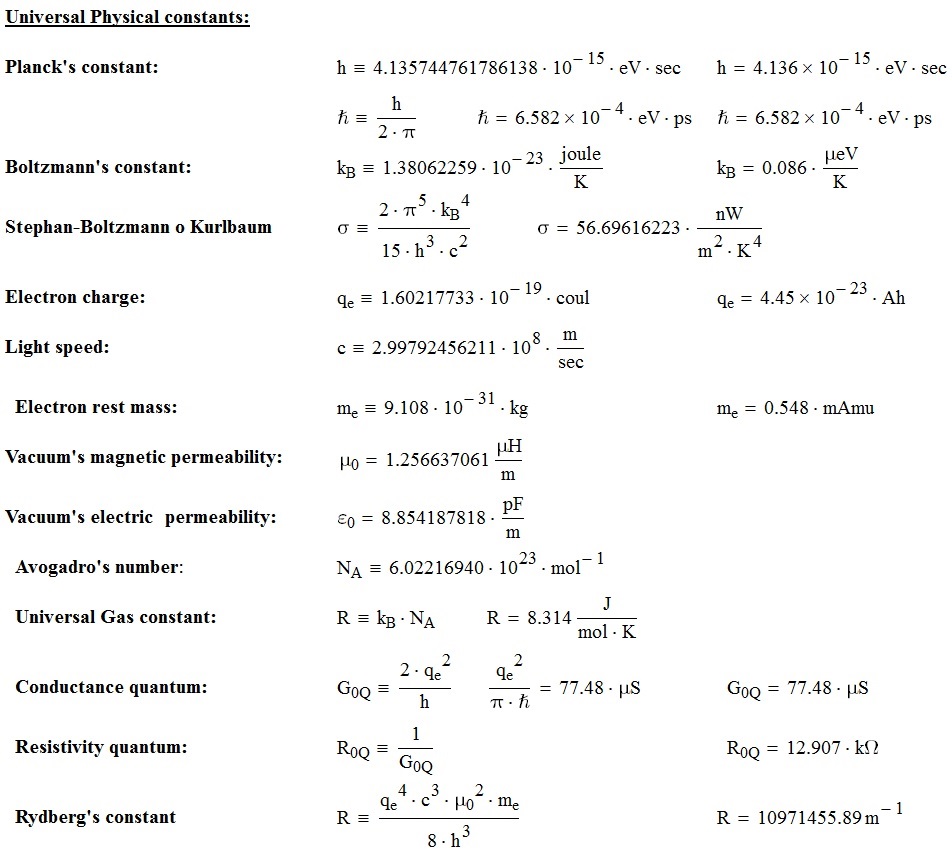
I would be very grateful if you find any imperfections in the previous list. Thank you very much
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Presently the speed of light is defined as (exactly!): 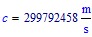 and
and
the unperturbed ground state hyperfine transition frequency of the caesium 133 atom is (exactly): 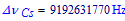
As of (sometime) next year (see http://www.bipm.org/utils/common/pdf/si-brochure-draft-2016b.pdf)
the following definitions will apply (and they will all be exact; their final exact values need to be determined, but they will be close to):
Plank constant:
Electron charge: 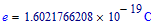
Boltzmann constant: 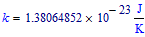
Avogadro constant: 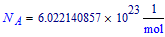
The luminous efficacy Kcd of monochromatic radiation of frequency 540 ×10^12 hertz: 
Further is determined (not exact):

With those, other natural constants become:

Luc
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Correction, the constant Kcd (luminous efficacy...) already exists since 1980.
The definition of the second (over the frequency of emission of a caesium atom) exists since 1964, that of the speed of light since 1984.
Luc
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
In Mathcad 15, R is a Rankine (unit of temperature that's equal to 5/9 Kelvin)
In Mathcad Prime 4, R can mean Rydbergs constant or the universal gas constant ( I don't think Prime knows Rankine temperature units)
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
I apologize, I did some confusion ...
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator

- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
I mistakenly called the perfect gas constant, R, with the name of another constant, that is, Rydberg. Also, while Prime supplies the correct R value (constant of perfect gases) (written with the label constant). This does not happen in mathcad 15, ie when writing R = mathcad provides a temperature.
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
So I'd never noticed R was built-in before this thread, and had always defined it myself. So I did a little comparison and found something interesting. The mathcad (Prime 3.1) R value does not appear to be correct.

- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
That's the problem with many of those natural constants. They change all the time.
Fortunately we'll be rid of many of that sometime next year. Then R (gas constant) will be exactly known.
It's a good thing you always defined R yourself.
Luc