Community Tip - Did you get an answer that solved your problem? Please mark it as an Accepted Solution so others with the same problem can find the answer easily. X
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
Find the temperature at which the concentration is maximum
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Find the temperature at which the concentration is maximum
Hello! I'm new to MathCad and I'm having trouble solving a kinetics and reactor calculus problem.
I have to find the temperature at which the concentration of B reaches its maximum value. I did this by manually guessing values, but I was wondering if it's possible to do it in an easier way.
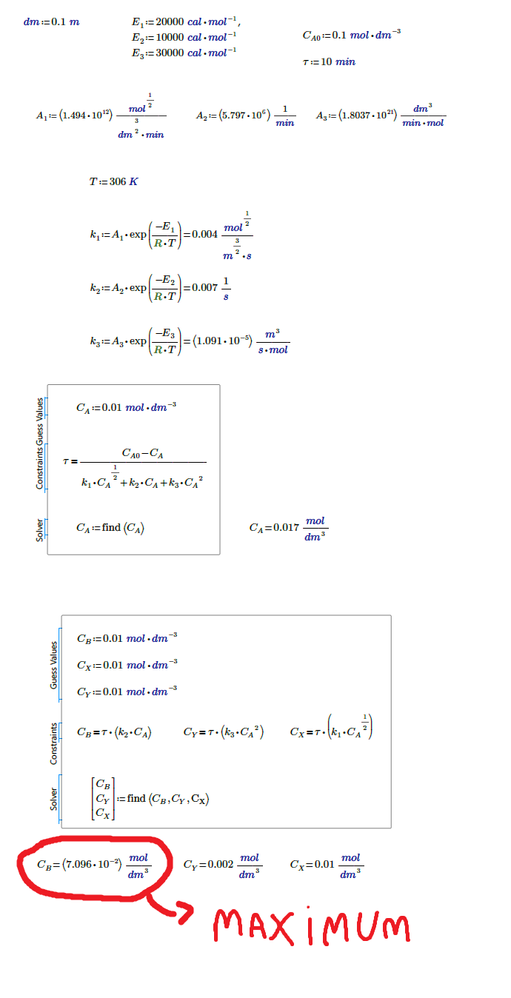
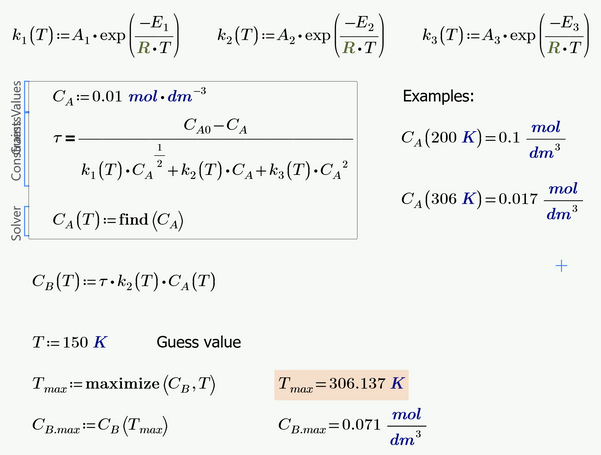
The constants k depend on the temperature. The concentration of A depends on the constants k. And finally, the concentration of B depends on the constants k and the concentration of A. The temperature I found was 306K. Would it be possible to synthesize all these calculations in a solve block? The following is the resolution and the way I was trying.
Solved! Go to Solution.
- Labels:
-
Chemical_Engineering
-
Mathcad Usage
Accepted Solutions
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Please attach your Prime sheet. Its way harder to debug a pic than a live worksheet.
I see no "B" nor any equation for the concentration of B in your pics.
Furthermore its irritation that your various k and A values are of different dimensions (units)
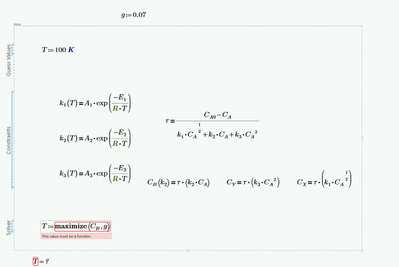
And then: Whats the reason for using a solve block to calculate the three C-values? It looks like you can define them directly via assignments.
> The concentration of A depends on the constants k.
It looks like its the other way round. In your pics you first define A's (different units!?) and then k dependent on a (and temp).
As I see it you will have to make k.1,2,3 functions of T and also turn the solve block for C.A into a function of T.
Then you can turn C.B into a function of T and find its maximum.
Similar to what you tried in your second pic but it looks like here the calculation of C.A is missing.
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
It's question f), ignore the other questions.
To find the concentration of B you would need to find the constant k2 and the concentration of A (Ca), the formula (τ*k2*Ca), whose variables depend on temperature. The parameters A are the pre-exponential factors of the Arrhenius equation, they have different units because the constants k also have different units (different order reactions). I would like to know a way to synthesize all these calculations. I don't know how to use the maximize function.
Anyway, thanks for the help!
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Thanksss!!