Community Tip - You can Bookmark boards, posts or articles that you'd like to access again easily! X
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
Question on determining sulphur flue gas temperature with given-find block
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Question on determining sulphur flue gas temperature with given-find block
Hi, everybody.
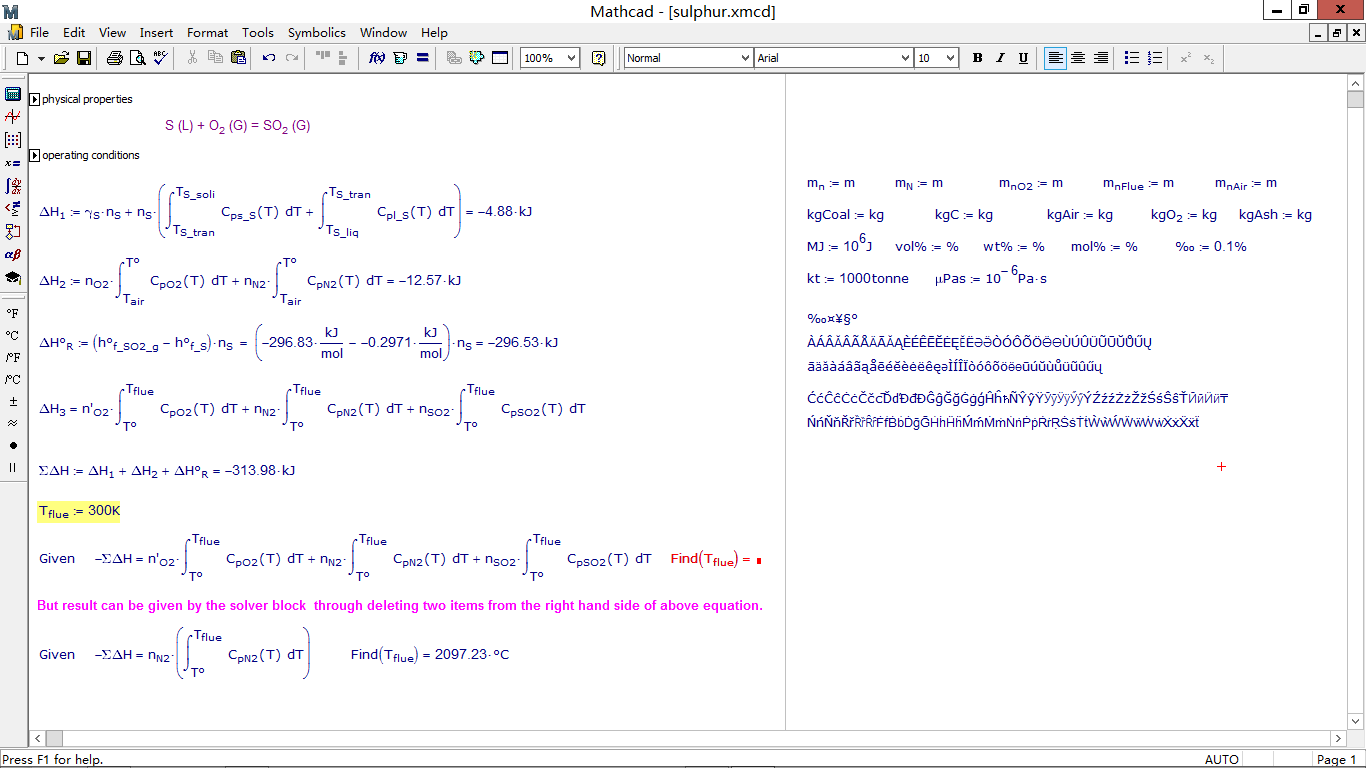
I met with an issue on solving for flue gas temperature which is the upper limit of an integral within an equation.
but when I deleted some items in the equation, the answer can be given by the solver block.
I m confused with this for a couple of days. Is this caused by the unit system? It seems that using dimensionless numbers takes effect, but this is not very convenient.
The attached file is in mathcad 15 format.
Does anybody give any suggestion on this problem?
Solved! Go to Solution.
- Labels:
-
Calculus_Derivatives
Accepted Solutions
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
When something like this happens, you should always plot the function(s) in question to see how they behave. In this case the solve block fails simply because there is no solution:

- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
When something like this happens, you should always plot the function(s) in question to see how they behave. In this case the solve block fails simply because there is no solution:

- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Hi, Richard
thanks for your advice, and i double checked my "physical properties" section. I found that there are two input mistakes in thermodynamic property definition, one is the sign of sulphur formation enthalpy, and the other is the power of temperature item in lastitem of Cp_so2 expression. After the correction, I got a reasonable answer.
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Hi FAN CG
I suppose that you, with H, meant to indicate the enthalpy. I do not know the details, but if, from the calculation, the enthalpy is negative, why, in the equation, you change the sign? If you do not change the sign, The equation gives a result, otherwise, as observed R.J., there are no solutions.
With best regards
FM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Thanks a lot for your help, F.M.
I input wrongly some thermodynamic parameters in my worksheet. and now I have corrected them.
the negative H is due to the fact that I integrated the capacity for higher temperature to low temperature to describe temperature drop.





