Turn on suggestions
Auto-suggest helps you quickly narrow down your search results by suggesting possible matches as you type.
Showing results for
Turn on suggestions
Auto-suggest helps you quickly narrow down your search results by suggesting possible matches as you type.
Showing results for
Community Tip - Need to share some code when posting a question or reply? Make sure to use the "Insert code sample" menu option. Learn more! X
Options
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
Boiling Point Elevation
Jul 14, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 14, 2009
03:00 AM
Boiling Point Elevation
Collab,
I am trying to calculate the elevation in boiling point as glycerol is added to water.
On the attached worksheet, I attempted to work from an online example by have a problem with the units.
Also, the expressions do not allow for very high concentration of glycerol in the calculation.
Any comments would be appreciated.
Thanks,
Art
I am trying to calculate the elevation in boiling point as glycerol is added to water.
On the attached worksheet, I attempted to work from an online example by have a problem with the units.
Also, the expressions do not allow for very high concentration of glycerol in the calculation.
Any comments would be appreciated.
Thanks,
Art
Labels:
- Labels:
-
Other
21 REPLIES 21
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 15, 2009
03:00 AM
1.Incorrect units.
Temperature elevation constant must be 0.521 C/mol/kg
Viktor Korobov
Temperature elevation constant must be 0.521 C/mol/kg
Viktor Korobov
Viktor
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 15, 2009
03:00 AM
... and water boiling point (at normal pressure) is not 100K but 373.15K (100C)
Val
http://twt.mpei.ac.ru/ochkov/v_ochkov.htm
Val
http://twt.mpei.ac.ru/ochkov/v_ochkov.htm
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 15, 2009
03:00 AM
1. Incorrect units.
Temperature elevation constant must be 0.521 C/mol/kg [Viktor]
2. ... and water boiling point (at normal pressure) is not 100K but 373.15K (100C) [Val]
3. ... boiling point(s) are function of absolute pressure
... the formulation does not look correct [jmG]
Temperature elevation constant must be 0.521 C/mol/kg [Viktor]
2. ... and water boiling point (at normal pressure) is not 100K but 373.15K (100C) [Val]
3. ... boiling point(s) are function of absolute pressure
... the formulation does not look correct [jmG]
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 15, 2009
03:00 AM
Thanks All,
Viktor's unit correction seemed to be the issue.
There still is an issue as the concentration of glycerol approaches 100%. Does anyone have any thoughts on that?
Art
Viktor's unit correction seemed to be the issue.
There still is an issue as the concentration of glycerol approaches 100%. Does anyone have any thoughts on that?
Art
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 15, 2009
03:00 AM
On 7/15/2009 12:11:05 PM, ArtZ wrote:
>Thanks All,
>
>Viktor's unit correction
>seemed to be the issue.
>
>There still is an issue as the
>concentration of glycerol
>approaches 100%. Does anyone
>have any thoughts on that?
>
>Art
_______________________________
The issue is that the formulation is incorrect. A problem of same nature as: how much to mix component of x% with component of y% for a resulting z%.
jmG
>Thanks All,
>
>Viktor's unit correction
>seemed to be the issue.
>
>There still is an issue as the
>concentration of glycerol
>approaches 100%. Does anyone
>have any thoughts on that?
>
>Art
_______________________________
The issue is that the formulation is incorrect. A problem of same nature as: how much to mix component of x% with component of y% for a resulting z%.
jmG
Jul 15, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
On 7/15/2009 12:11:05 PM, ArtZ wrote:
>Thanks All,
>
>Viktor's unit correction
>seemed to be the issue.
>
>There still is an issue as the
>concentration of glycerol
>approaches 100%. Does anyone
>have any thoughts on that?
>
>Art
One picture for this subject:
Val
http://twt.mpei.ac.ru/ochkov/v_ochkov.htm
>Thanks All,
>
>Viktor's unit correction
>seemed to be the issue.
>
>There still is an issue as the
>concentration of glycerol
>approaches 100%. Does anyone
>have any thoughts on that?
>
>Art
One picture for this subject:
Val
http://twt.mpei.ac.ru/ochkov/v_ochkov.htm
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
Glycerol-water mixtures: Boiling points in attached.
Viktor Korobov
Viktor Korobov
Viktor
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
..and saved in MC11 format.
Viktor Korobov
Viktor Korobov
Viktor
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
On 7/16/2009 9:26:13 AM, vikkor wrote:
>..and saved in MC11 format.
>Viktor Korobov
____________________________
Tanks Viktor,
Recreated in 11,
as your saved 11 version is scraped
but could be recuperated !
jmG
>..and saved in MC11 format.
>Viktor Korobov
____________________________
Tanks Viktor,
Recreated in 11,
as your saved 11 version is scraped
but could be recuperated !
jmG
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
Jean,
Thanks for recovering the file. I have MC2001i and MC13 and couldn't read Viktor's files at all.
Did Viktor cite his source of data in his postings?
If the glycerol mole fraction is 100% wouldn't that represent the BP of 290�C? Seems the x scale is reversed.
The funny step as the glycerol mole fraction --> 100% doesn't seem correct. I'd expect a smooth transition.
Since I am working in mass fraction, I'll need to convert from mole fraction.
Art
Thanks for recovering the file. I have MC2001i and MC13 and couldn't read Viktor's files at all.
Did Viktor cite his source of data in his postings?
If the glycerol mole fraction is 100% wouldn't that represent the BP of 290�C? Seems the x scale is reversed.
The funny step as the glycerol mole fraction --> 100% doesn't seem correct. I'd expect a smooth transition.
Since I am working in mass fraction, I'll need to convert from mole fraction.
Art
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
On 7/16/2009 1:41:21 PM, ArtZ wrote:
>Jean,
>
>Thanks for recovering the
>file. I have MC2001i and MC13
>and couldn't read Viktor's
>files at all.
>
>Did Viktor cite his source of
>data in his postings?
>
>If the glycerol mole fraction
>is 100% wouldn't that
>represent the BP of 290�C?
>Seems the x scale is reversed.
>
>The funny step as the glycerol
>mole fraction --> 100% doesn't
>seem correct. I'd expect a
>smooth transition.
>
>Since I am working in mass
>fraction, I'll need to convert
>from mole fraction.
>
>Art
Sorry, see revised variant.
Viktor Korobov
>Jean,
>
>Thanks for recovering the
>file. I have MC2001i and MC13
>and couldn't read Viktor's
>files at all.
>
>Did Viktor cite his source of
>data in his postings?
>
>If the glycerol mole fraction
>is 100% wouldn't that
>represent the BP of 290�C?
>Seems the x scale is reversed.
>
>The funny step as the glycerol
>mole fraction --> 100% doesn't
>seem correct. I'd expect a
>smooth transition.
>
>Since I am working in mass
>fraction, I'll need to convert
>from mole fraction.
>
>Art
Sorry, see revised variant.
Viktor Korobov
Viktor
Jul 16, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 16, 2009
03:00 AM
Art,
This work sheet 2001i, is Viktor in mol fraction,
with a slight correction on the 'x' marker.
Jean
This work sheet 2001i, is Viktor in mol fraction,
with a slight correction on the 'x' marker.
Jean
Jul 17, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 17, 2009
03:00 AM
Jean/Viktor,
On the attached worksheet, I have converted the mole fraction values from Viktor to mass fractional.
I was surprised to find that the relationship between mass fraction and boiling point is likely linear.
Viktor, where did you get the mole fraction values versus boiling point.
Please let me know if I didn't do the conversion correctly.
Thanks,
Art
On the attached worksheet, I have converted the mole fraction values from Viktor to mass fractional.
I was surprised to find that the relationship between mass fraction and boiling point is likely linear.
Viktor, where did you get the mole fraction values versus boiling point.
Please let me know if I didn't do the conversion correctly.
Thanks,
Art
Jul 17, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 17, 2009
03:00 AM
>I was surprised to find that the relationship between mass fraction and boiling point is likely linear<<br> _________________________
The question is really what are you looking for ?
Jean
The question is really what are you looking for ?
Jean
Jul 17, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 17, 2009
03:00 AM
Jean,
I am using glycerol/water mixtures as a stable, variable thermal load.
You may recall that previously, a worksheet with thermal conductivity versus mass fraction was posted.
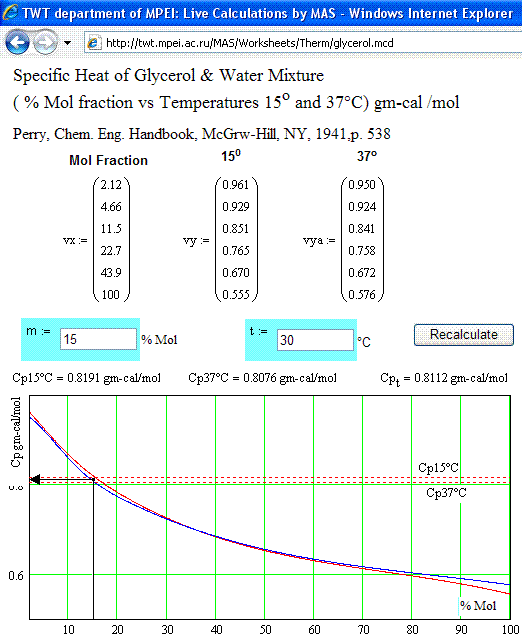
Recently, I obtained data on heat capacity versus glycerol mass fraction.
With the addition of the boiling point versus mass fraction, I can customize my thermal loads for all three parameters.
Art
I am using glycerol/water mixtures as a stable, variable thermal load.
You may recall that previously, a worksheet with thermal conductivity versus mass fraction was posted.
Recently, I obtained data on heat capacity versus glycerol mass fraction.
With the addition of the boiling point versus mass fraction, I can customize my thermal loads for all three parameters.
Art
Jul 17, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 17, 2009
03:00 AM
On 7/17/2009 5:56:23 PM, ArtZ wrote:
>Jean,
>
>I am using glycerol/water
>mixtures as a stable, variable
>thermal load.
>
>You may recall that
>previously, a worksheet with
>thermal conductivity versus
>mass fraction was posted.
>
>Recently, I obtained data on
>heat capacity versus glycerol
>mass fraction.
>
>With the addition of the
>boiling point versus mass
>fraction, I can customize my
>thermal loads for all three
>parameters.
>
>Art
_____________________________
Art,
I'm trying to understand that given a volume of glycol p2 you want the temperature of the mixture as function of the variable p1 volume of water.

Does it make sense if you put correct b1, b2 as per handbooks ? The linearity depends upon b1, b2 (which I don't have off hand).
Jean
>Jean,
>
>I am using glycerol/water
>mixtures as a stable, variable
>thermal load.
>
>You may recall that
>previously, a worksheet with
>thermal conductivity versus
>mass fraction was posted.
>
>Recently, I obtained data on
>heat capacity versus glycerol
>mass fraction.
>
>With the addition of the
>boiling point versus mass
>fraction, I can customize my
>thermal loads for all three
>parameters.
>
>Art
_____________________________
Art,
I'm trying to understand that given a volume of glycol p2 you want the temperature of the mixture as function of the variable p1 volume of water.

Does it make sense if you put correct b1, b2 as per handbooks ? The linearity depends upon b1, b2 (which I don't have off hand).
Jean
Jul 18, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 18, 2009
03:00 AM
Art,
I think your mole/mass conversion is wrong. See attach.
Viktor Korobov
I think your mole/mass conversion is wrong. See attach.
Viktor Korobov
Viktor
Jul 20, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 20, 2009
03:00 AM
Viktor,
Thanks for your post; that is a lot work! I am just now looking at your worksheet and don't completely follow what you have done.
From your work, it appears that boiling point is very non-linear.
I may have asked you in an earlier post and you may have responded, however, could you name the source of your original boiling point versus mole fraction data?
Thanks again,
Art
Thanks for your post; that is a lot work! I am just now looking at your worksheet and don't completely follow what you have done.
From your work, it appears that boiling point is very non-linear.
I may have asked you in an earlier post and you may have responded, however, could you name the source of your original boiling point versus mole fraction data?
Thanks again,
Art
Jul 20, 2009
03:00 AM
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Notify Moderator
Jul 20, 2009
03:00 AM
>versus mole fraction data<<br>
That complicates matter, just go by my formula. It does the mole fraction algebraically, look at the formula again where you simply put the weight or the mass if you prefer. The formula is the "homographic function", one form of it, an expanded form of 1/x. For sure it is not linear, it is of the reciprocal form, i.e: a rational fraction. Also, you must understand that you need the specific heat capacity of each component.
Just reproduce the attached, fill-in the respective data.
Jean
That complicates matter, just go by my formula. It does the mole fraction algebraically, look at the formula again where you simply put the weight or the mass if you prefer. The formula is the "homographic function", one form of it, an expanded form of 1/x. For sure it is not linear, it is of the reciprocal form, i.e: a rational fraction. Also, you must understand that you need the specific heat capacity of each component.
Just reproduce the attached, fill-in the respective data.
Jean



